Two-Year Follow-Up of NOVOGLAN-01 Open Label Multicentre Clinical Trial: Efficacy and Safety of Novoglan for Adult Phimosis
Aim:
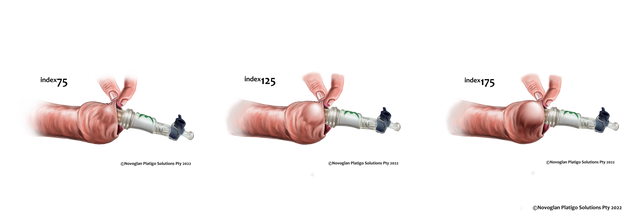
Conventional definitive treatment for adult phimosis is circumcision. Novoglan device is a
potential non-surgical alternative through the application of custom-molded balloons for
gradual skin remodeling and prepuce dilation. Initial results of the NOVOGLAN-01 open label
clinical trial demonstrated promising outcomes. This follow-up abstract presents the two-year
results, aimed at establishing long-term efficacy, safety, and tolerability.
Methods:
A prospective, open-label trial was conducted on the first 20 adult patients with phimosis at
Macquarie University Hospital and Princess Alexandra Hospital. Following eligibility
screening, patients underwent Novoglan treatment involving twice-daily 10-minute
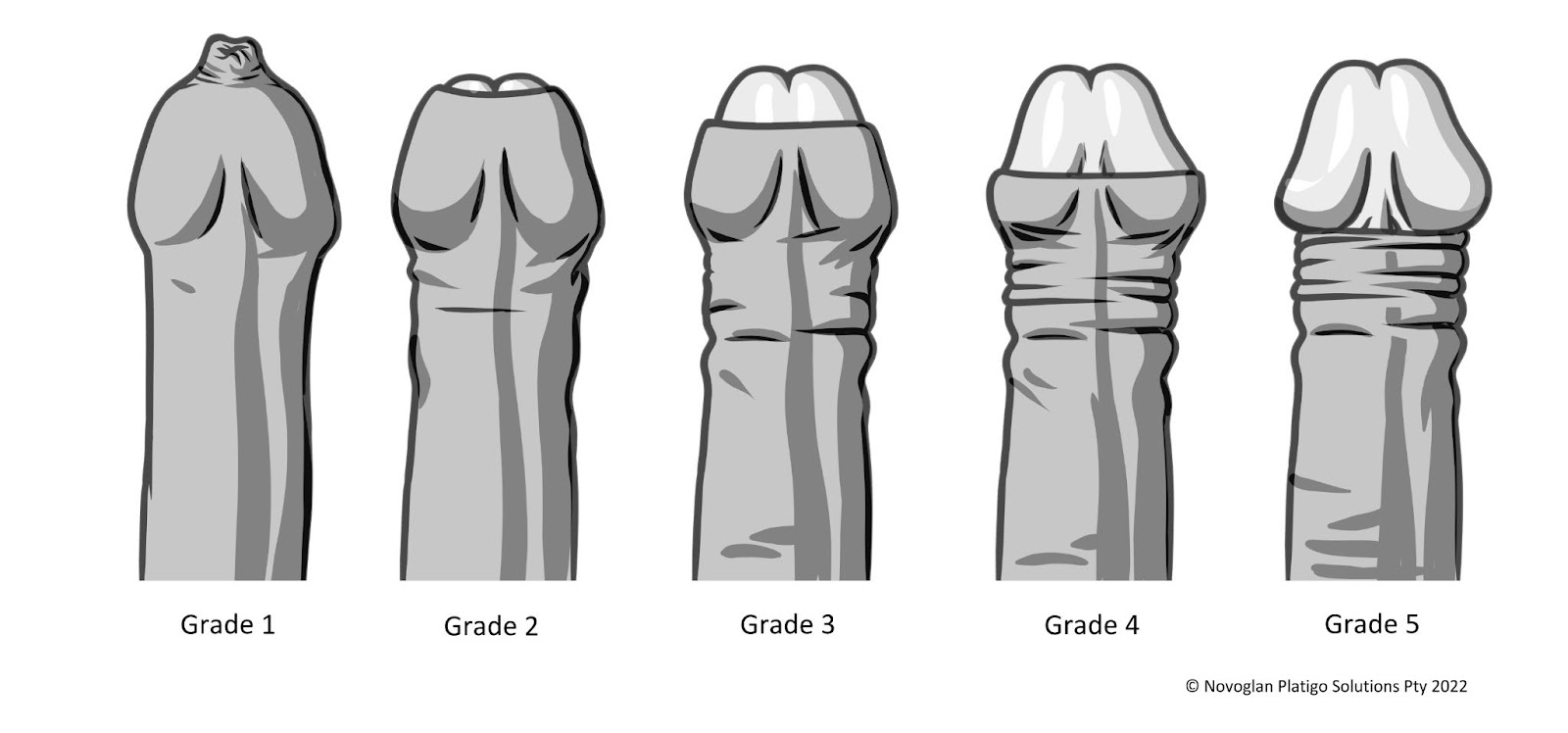
applications for 4-8 weeks. Efficacy was measured by the degree of phimosis before and
after treatment, with follow-ups at 6-8 weeks and two years. Each patient served as their
own control.
Results:
Two-year follow-up data revealed that 19 of the first 20 patients (95%) maintained
successful outcomes with full normal retraction. Among these 19 patients, 100% reported
no new symptoms and did not require further intervention or circumcision. The statistical
analysis supports that these results are likely attributable to the Novoglan treatment rather
than chance, given the consistent improvement across individual cases and sustained
efficacy over two years.
Conclusions:
The NOVOGLAN-01 trial’s two-year follow-up data underscore the long-term efficacy and
safety of Novoglan for treating adult phimosis. Each patient serving as their own control
further strengthens the validity of these results. The high success rate, absence of new
symptoms, and lack of need for further surgical intervention highlight the potential of
Novoglan as a viable non-surgical alternative to circumcision.
References:
Trial registration:
The NOVOGLAN-01 study is registered with the Australia and New Zealand Clinical Trial
Registry under reference ACTRN 1262 10009 24853, dated 15 July 2021.
Authors: E. Chung, D. Polikarpov, H. Mazure, A. James, H. Doosti, D. Campbell, D. Gillatt